The Handling and Reduction of SOx, NOx and SPM
The growing menace of pollution across the globe has become a major concern for the life on Mother Earth. While the powers are trying to focus and minimize it, an equal onus lies on the population at large. Onus whether one is an Industrialist or workman.
Of the many sources of pollution, the use of fossil fuels plays a major role in it. Automobiles, Three Wheelers, Two Wheelers, Locomotives. Industrial usage all contribute to it.
This article aims to address the pollution arising out of Industrial usage of Light Diesel Oil, LSHS, Heavy Oil, LPG, Natural Gas etc. for the express purpose of generating heat in ovens, furnaces, boilers, thermal oil heaters, hot water generators, Drying systems etc.
If not always, all the above applications most of the time use the above fuels to generate heat through burners or any other firing mechanism.
The major pollutants arising out of the combustion of these fuels are:
- Sulphur Oxides, denoted by SOx and mainly comprising of Sulphur Dioxide SO2 and Sulphur Trioxide SO3
- Nitric Oxides, denoted by NOx and comprising of Nitric Oxide NO and Nitrogen Dioxide NO2
- Carbon Monoxide CO
- Carbon Dioxide CO2
- Total Suspended Particles indicated by TSP or SPM.
All the pollutants adversely affect human health. And each of the above pollutants has its own share in impacting human health
Sulphur Oxides:
Sulphur Dioxide is a colourless gas with a density equal to nearly two and a half times that of air. Thus, it tends to settle down towards the ground in closed environments.
Sulphur Oxides are found to be toxic for humans; especially Sulphur Dioxide SO2 causes irritation of the eyes and watering of eyes (lachrymation) when the concentration exceeds 300 mg/Nm3. The danger threshold is estimated at around 500 mg/Nm3.
Formation:
Moderate temperatures favour the formation of Sulphur Oxides. Under normal conditions of high combustion flame temperature and excess air around 20%, nearly all the Sulphur present in the fuel Oxidizes into Sulphur Dioxide (SO2).
The percentage of Sulphur Trioxide SO3 would be important for low combustion temperatures (400°C), during start-up phases of installations, when the excess air is extremely high or if pure oxygen is used.
Sulphur Trioxide SO3reacts with water vapour, generating Sulphuric Acid H2SO4 which is corrosive even in the vaporous phase, thereby damaging the heat exchangers/ generators, which are generally metallic.
Nitric Oxides:
Nitric Monoxide NO is a colourless, odourless gas that is insoluble in water.
More than 90% of all nitric oxides formed during high-temperature combustion processes are represented by Nitric Oxide.
Nitrogen Dioxide NO2is a visible gas even in low concentrations, with a brownish-reddish colour and acrid smell. It is highly corrosive and an irritant to the nasal membranes and eyes in the concentration range of 10 ppm, it causes bronchitis at concentrations of 150 ppm and pulmonary oedema at 500 ppm, even if the exposure is limited to a few minutes.
Nitric Monoxide NO present in the air can transform itself into Nitrogen Dioxide NO2by means of photochemical oxidation.
Formation:
Three models of nitric oxide formation exist, which lead to the formation of different types of nitric oxide (different by type of origin but not by chemical composition); respectively they are:
- Thermal Nitric Oxides (Thermal NOx)
- Prompt Nitric Oxides (Prompt NOx)
- Fuel Nitric Oxides (Fuel NOx)
Formation of Thermal Nitric Oxides is due to the oxidation of atmospheric nitrogen (contained in combustion air) under high temperature (T>1500 K) and high oxygen concentration conditions. They represent most of the Nitric oxide in the case of gaseous fuels (Methane and LPG) and in general in fuels that do not contain nitrogenous compounds.
Fixation of atmospheric nitrogen by hydrocarbon fragments (radicals) present in the flame area results in the formation of Prompt Nitric Oxides. This method of forming oxides is extremely rapid thus giving rise to the name prompt.
This formation principally depends on the concentration of radicals in the first stage of the flame. In the case of oxidative flames (combustion with an excess of oxygen), their contribution is negligible, while in the case of rich mixtures and for low-temperature combustion, their contribution could reach 25% of the full nitric oxides total.
Nitric Oxides from fuel form by means of oxidation of the nitrogenous compounds contained in the fuel within the flame area. Their production is significant when the fuel’s nitrogen content exceeds 0.1% in weight, primarily and only for liquid and solid fuels.
Depending on the type of fuel and considering standard combustion conditions, the portion of Prompt Nitric Oxides remains almost constant, whereas the portion of Fuel Nitric Oxides grows, and the portion of Thermal Nitric Oxides decreases as we gradually pass to fuels with a higher molecular weight.
Carbon Monoxide:
Carbon Monoxide is a colourless, odourless and tasteless gas. Its relative density compared to air is 0.96, and thus it does not disperse easily.
Carbon Monoxide is a toxic gas that, if inhaled, reacts extremely rapidly with the Hemoglobin in the blood, preventing the regular oxygenation of the blood and, as of the entire organism. The physiological effects on the organism are the result of the concentration of the carbon monoxide in the air and the length of exposure of the person to said concentration.
Formation:
The partial oxidation of the carbon present in the fuel gives rise to the formation of Carbon Monoxide. The presence of Carbon Monoxide in flue gases indicates low combustion efficiency as the carbon is not perfectly oxidized to form CO2 and corresponds to heat not produced.
Combustion with low quantity air (Low when compared to Stoichiometric requirements) results in the generation of Carbon Monoxide in flue gases as insufficient oxygen is available to complete the Carbon Oxidation reactions.
Carbon Dioxide:
Of the many products, the main products of any hydrocarbon combustion process are Carbon Dioxide and Water Vapour.
The Greenhouse Effect is a result of the accumulation of Carbon Dioxide in the atmosphere. Light arriving from our Sun passes through Earth’s atmosphere and warms its surface. The warmed surface then radiates heat (infrared). Part of infrared radiation is absorbed by the Carbon Dioxide accumulated in the atmosphere thus retaining the heat. The result of this phenomenon is the progressive increase in the Earth’s average temperature with disastrous resulting consequences.
Formation:
The quantity of carbon C present in the fuel determines the absolute Carbon Dioxide quantity produced by combustion.
The greater the C/H ratio of the fuel, the greater the quantity of Carbon Dioxide produced will be.
Liquid fuels produce more Carbon Dioxide than gaseous fuels.
For better control of combustion, the percentage of CO2 in the flue gases must be as high as possible to achieve greater output.
A lower CO2 percentage in the combustion flue gases leads to the system being less efficient, resulting in oxidation of more fuel.
| FUEL | CO2 max | CO2 | Excess Air |
| in vol [%] | Advised [%] | [%] | |
| METHANE | 11.65 | 9.8 – 11 | 20 – 10 |
| L.P.G. | 13.74 | 11.5 – 12.8 | 20 – 10 |
| TOWN GAS | 10.03 | 8.2 – 9 | 30 – 12 |
| LIGHT OIL | 15.25 | 12 – 14 | 35 – 20 |
| HEAVY OIL | 15.6 | 11.8 – 13 | 35 – 20 |
Total Suspended Particles/Suspended Particulate Matter:
Emissions comprising of particulates, inert solid substances and metallic components are included in this category.
The size of these particles varies from a minimum of 0.01 microns up to a maximum of 500 microns. These can be of an organic or inorganic nature. Broadly these are categorised as follows:
- Ashes, comprising inorganic, incombustible substances (metals, etc.)
- Gas black, made up of the fuel residues which have evaporated but not oxidised
- Cenopheres, comprising fuel residues that have been partially oxidised since they have been burnt before vaporising.
The finest portion of the particulate is called soot.
The danger of the particles is inversely proportionate to the size. Damage caused is mainly to the respiratory tracts and pulmonary system.
The depth these particles can penetrate the human body depends on their size.
Furthermore, in the pulmonary alveolus the particulate acts as the vehicle transporting the metallic oxides (vanadium, nickel etc.) which may be produced during combustion, and which are absorbed by the particles of the particulate.
Only the particles with an equivalent diameter smaller than 10 microns are sufficiently light to remain suspended in the air for several hours and therefore represent real danger of being inhaled.
Formation:
Metal oxide emissions depend on the concentration of the respective metals in the fuel. The best solution for reducing emission requires use of fuels with low heavy metals concentrations.
Gas black is usually produced when the Oxygen is insufficient or low temperature conditions within the flame. To avoid the formation of gas black during combustion, it is necessary to ensure an adequate temperature, enough oxygen and considerable turbulence in order to obtain a satisfactory mix between the fuel and the oxygen.
Cenospheres form when the nebulization and volatilization process of the liquid fuels in the combustion chamber is irregular or hindered by the elevated viscosity and low volatility of the fuel.
In order to reduce the production of these components, it is necessary to increase the period spent in the combustion chamber and guarantee the fuel an adequate excess of oxygen.
Having said that, let’s look at Combustion in a Burner, how the pollutants are formed and how the same can be minimised.
Combustion:
The rapid oxidation of fuel is termed as Combustion. The reaction is accompanied by that visible physical phenomenon which is called ‘flame’ and by the generation of energy that is known as ‘heat’.
Carbon combines with Oxygen to form Carbon Dioxide, a non-toxic gas, and releases heat according to the following formula:
C + O2 → CO2 + Heat
Similarly, Hydrogen combines with Oxygen to form Water Vapour, resulting in production of heat, according to the following formula:
2H2 + O2 → 2H2O + Heat
It is important to note that fuel and Oxygen combine in well-defined and specific proportions. The quantities of Oxygen and fuels in the mixture are in perfect or “Stoichiometric” proportion, when they enable complete oxidation of the fuel without any Oxygen residue.
If there is an excess of fuel or insufficient Oxygen, the mixture is rich, and the flame is reducing. This is defined as incomplete combustion. Though certain fuel particles are completely oxidised by the Oxygen, others do not receive enough oxygen and consequently their combustion is only partial.
Partial or incomplete carbon combustion is accompanied by the formation of Carbon Monoxide, a highly toxic gas as depicted by the following reaction formula:
2C+O2 →2CO + Heat
The amount of heat produced here is lower than that produced by perfect combustion.
If excessive Oxygen is supplied to the mixture, the mixture is weak, and combustion is oxidative.
Besides Carbon Dioxide and Water Vapour, other compounds are produced during combustion in smaller amounts, such as Sulphur Oxides, Nitric Oxides, Carbon Monoxide, Carbon Dioxide and metallic oxides as already described above.
The oxidative gas normally used is air, which is a gas mixture mainly made up of Oxygen and Nitrogen.
If the exact chemical composition of the fuel is known, the stoichiometric amount of oxygen and consequently the combustion air required for combustion purposes can be calculated.
However, the quantity of air derived from the calculations is theoretical. The composition of air varies from season to season, location to location, altitude to altitude and so on so forth. Moreover, a production facility cannot calculate the exact air and run the plant continuously without changing the quantity of air based on composition analysis.
Thus, in all the combustions of these types, excess air is used. The quantity of excess air required varies from fuel to fuel. Lowest amount of excess air is required for gaseous fuels while highest for solid or hard to combust fuels.
Excess air is used to ensure the availability of enough air and thus by extension of oxygen for proper and nearly complete combustion.
It should be noted here that, though excess air ensures proper and/or complete combustion, it also absorbs some amount of heat (infinitesimal when compared to the improved combustion) generated which does not contribute to the output process heat.
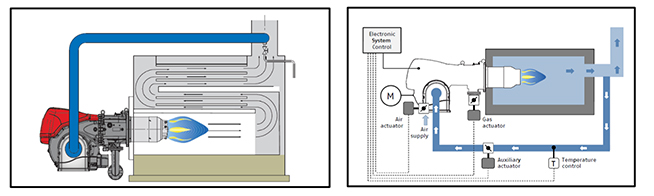
Burner:
The term burners describes a series of equipment for burning various types of fuel under suitable conditions for perfect combustion. The burner operates by sucking in the fuel and the combustion supporter air, mixes them thoroughly together and safely ignites them inside the heat generator furnace.
The following are the parts that make up the burner and are analysed individually in the following paragraphs.
- The combustion head which mixes the fuel and the combustion supporter, and generates an optimum form of flame
- The combustion air supply, comprising of the fan and any pipes for taking the air to the combustion head
- Fuel supply, comprising components used for regulating the fuel flow and guaranteeing the safety of the combustion system
- The electrical and control components required for firing the flame, the electricity supply to the motors and thermal output regulation developed by the burner.
Modern burners are designed on the one hand to deliver higher combustion efficiencies and on the other reduce pollutants.
This is achieved by acting on the process and combustion equipment (burner), to ensure that combustion takes place under the best conditions thus reducing the formation of pollutants.
The firing range of any burner is deduced through special test boilers according to methods established by European legislation, particularly:
o EN 267 standard for liquid fuel burners
o EN 676 standard for gaseous fuel burners
These standards establish the dimensions that the test combustion chamber must have.
Burners can be extremely sensitive to combustion chamber architecture and combustion chamber draught:
- Greater is the draught, greater is the amount of air sucked in and mixed with the gaseous fuel
- Too low Draught causes combustion without air, resulting in extremely dangerous pollutants such as CO.
Based on the above explanations, it is evident that however well a burner is designed, the performance of the burner in terms of Efficiency, Emissions etc. is controlled to a large extent by the Fuels used, Stoichiometric settings, Combustion Chamber design etc.
SOx, SPM, CO2, etc. may be influenced by the burner design to an extent. But NOX to a wide extent can be managed by Burner design.
The presence of nitrogenous compounds in liquid fuels is the source of NOx production representing a significant portion of the total NOx.
It has been found that, formation of nitric oxides from fuels in reducing environments the nitrogen contained in the fuel may not necessarily produce the undesired NOx, but simple and harmless molecular nitrogen N2.
The combustion chamber is an environment designed for the oxidation of fuels. Nevertheless, it is possible to create zones rich in fuel in certain regions of the flame and therefore form reducing situations for the purpose of producing molecular nitrogen N2 in the place of nitric oxides.
It is possible to supply the initial combustion region with 80% of the total combustion air together with 100% of the fuel and, further on, supply the remaining 20% of the combustion air (over firing air).
The thermal nitric oxides in gaseous fuels represent up to 80% of total emissions. However, a drop in the combustion temperature inhibits the formation of these compounds.
The temperature drop may be carried out in various ways – which is a design aspect of the burner.
In order to comply with the increasing demand of very low NOx emissions, Riello as a pioneer spanning 100 + years of design experience has introduced Low NOx and Ultra Low NOx Burners.
Schematic of design achieving Reduced Emissions in Riello Burners are as given below:
Reducing Flame Temperature:
The configuration of the combustion head results in internal re-circulation of the combustion substances. This re-circulation reduces the flame temperature and therefore the NOX emissions.
Additionally, the re-circulation of the combustion substances speeds up evaporation of combustible droplets creating gassy type combustion, like gas burner blue flame.
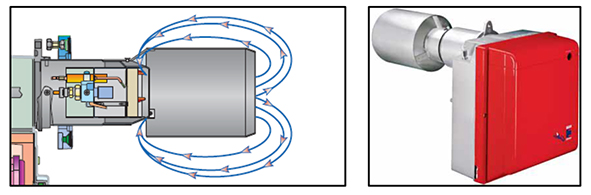
Flue Gas Recirculation (FGR):
FGR technology is based on the recirculation of a part of the exhaust gas, which is introduced in the air inlet side of the burner; an integrated Digital Burner Management System, trough the action of independent servomotors, allows the control of air, fuel and exhaust gas proportion in every working point, in order to reach very low NOx emissions, while maintaining high reliability of operation.
All the components are integrated in a compact size, so as to facilitate extremely easy installation and maintenance.
Typical Emission Range of Riello Burners:
| NOx emissions in mg/kWh (EN 676) – GAS | |||
| Class | N.G. (G20) | GPL | |
| 1 | ≤ 170 | ≤ 230 | Standard Burners |
| 2 | ≤ 120 | ≤ 180 | MZ Series |
| 3 | ≤ 80 | ≤ 140 | Low NOx BLU Series |
| ULN | ≤ 30 | NA | Ulra Low NOx |
| NOX – CO emissions in mg/kWh (EN 267) – OIL | |||
| Class | NOx | CO | |
| 1 | ≤ 250 | ≤ 110 | Standard Burners |
| 2 | ≤ 185 | ≤ 110 | MZ Series |
| 3 | ≤ 120 | ≤ 60 | Low NOx BLU Series |
The Series of Riello Burners designed for Low NOX and Ultra Low NOX are as follows:
RS/E FGR – DB SE FGR Series
Monoblock and Dual Block Gas FGR Burners
Ultra Low NOx Gas Burners – NOx < 30 mg/kWh (< 15 ppm)
Riello-Suntec offers an extensive and wide range of burners for all applications:
Capacity: 5 Kw to 80 MW.
Fuels: LDO, LSHS, Heavy Oil, NG, LPG, Lean Gases
Type: Single Stage, Two Stage, Modulating, High Modulating Ratio, Temperature Burners, Duct Burners etc.
Accessories: HPRT, Blowers, Control Panels, etc.
Disclaimer:
The contents of this article are based on various technical & open sources and the experience of the writer. The writers’ opinions are his own and do not constitute technical advice in any way whatsoever. Nothing published in this article constitutes a technical recommendation, nor should any data or content published in this article be relied upon for any technical activities.
The author strongly recommends that you perform your own independent research and/or speak with a qualified technical professional before making any technical decisions.
Rajesh Pathak, General Manager – Business Development, Suntec Energy Systems